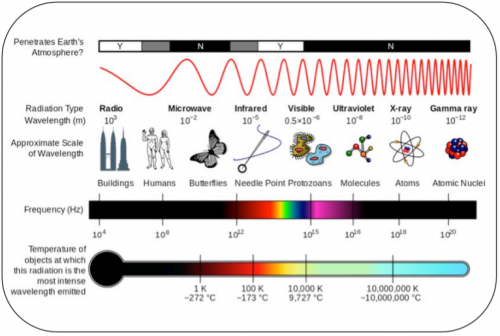
Electromagnetic Spectrum
Light as Waves
Physicists describe light as something called electromagnetic radiation or electromagnetic wave [1]. The word radiation means ‘energy that is transported from one spot to another without need of direct contact between the two locations’. Light in each of these colors carries a different amount of energy: gamma rays are the most energetic, and radio is the least. Thus, when we observe gamma or X-rays from an astronomical object, we know that something really powerful is happening there. This energy is transported in the form of a wave, and each color is related to a different wave size: The more energy the wave carries, the narrower it is –or, in technical terms, the shorter its wavelength. Hence, X-rays have shorter wavelengths than visible-light waves, and visible light has shorter wavelengths than radio waves.
Properties of Waves
A wave is a periodic perturbation from an undisturbed or rest state that is transmitted in space. The maximum deviation from this undisturbed state is called the amplitude of the wave, and is a measure of the intensity of the perturbation; the top of the wave is called a crest and the lowest state, a trough. Waves are characterized by their size, measured as the distance between two equal states; this distance is called the wavelength, [math]\displaystyle{ \lambda }[/math]. The colors of visible light are the way our eyes perceive the different wavelengths. The time it takes for the perturbation to move one wavelength –in other words, to repeat itself–, is called the period of the wave, [math]\displaystyle{ P }[/math]. Wavelength and period are related through the speed of the wave, [math]\displaystyle{ c }[/math] [math]\displaystyle{ (≈3*10^8 m/s) }[/math], because speed is defined as space travelled per unit time:
[math]\displaystyle{ c = \frac{\lambda}{P} }[/math]
The number of complete cycles –the number of times the wave repeats itself– in one second is called the frequency of the wave, [math]\displaystyle{ f }[/math]. Thus, the frequency is the inverse of the period, and is also related to the wavelength through the wave speed:
[math]\displaystyle{ f = \frac{1}{P} = \frac{c}{\lambda} }[/math]
It is important to note that the speed of light waves does not depend on the frequency, wavelength or period, but only on the medium the wave is traveling through. However, contrary to other waves like sound or water waves, electromagnetic waves (light) do not actually require a medium to be transmitted through: they can travel through the vacuum. If light travels through a vacuum, it has the maximum possible speed –and this speed is the same for all frequencies of light.
Sections of the Electromagnetic Spectrum
Infrared
Within the electromagnetic spectrum, infrared waves occur at frequencies above those of microwaves and just below those of red visible light, hence the name "infrared."[2] Similar to the visible light spectrum, which ranges from violet (the shortest visible-light wavelength) to red (longest wavelength), infrared radiation has its own range of wavelengths. The shorter "near-infrared" waves, which are closer to visible light on the electromagnetic spectrum, don't emit any detectable heat and are what's discharged from a TV remote control to change the channels. The longer "far-infrared" waves, which are closer to the microwave section on the electromagnetic spectrum, can be felt as intense heat, such as the heat from sunlight or fire. IR radiation is one of the three ways heat is transferred from one place to another, the other two being convection and conduction. Everything with a temperature above around 5 degrees Kelvin (minus 450 degrees Fahrenheit or minus 268 degrees Celsius) emits IR radiation. The sun gives off half of its total energy as IR, and much of the star's visible light is absorbed and re-emitted as IR.
Visible Light
The narrow visible part of the electromagnetic spectrum corresponds to the wavelengths near the maximum of the Sun's radiation curve.[3] In interactions with matter, visible light primarily acts to elevate electrons to higher energy levels. White light may be separated into its spectral colors by dispersion in a prism
Ultraviolet
The region just below the visible in wavelength is called the near ultraviolet. It is absorbed very strongly by most solid substances, and even absorbed appreciably by air. The shorter wavelengths reach the ionization energy for many molecules, so the far ultraviolet has some of the dangers attendant to other ionizing radiation. The tissue effects of ultraviolet include sunburn, but can have some therapeutic effects as well. The sun is a strong source of ultraviolet radiation, but atmospheric absorption eliminates most of the shorter wavelengths. The eyes are quite susceptible to damage from ultraviolet radiation. Welders must wear protective eye shields because of the uv content of welding arcs can inflame the eyes. Snow-blindness is another example of uv inflammation; the snow reflects uv while most other substances absorb it strongly.
X-Rays
X-ray was the name given to the highly penetrating rays which emanated when high energy electrons struck a metal target. Within a short time of their discovery, they were being used in medical facilities to image broken bones. We now know that they are high frequency electromagnetic rays which are produced when the electrons are suddenly decelerated - these rays are called bremsstrahlung radiation, or "braking radiation". X-rays are also produced when electrons make transitions between lower atomic energy levels in heavy elements. X-rays produced in this way have have definite energies just like other line spectra from atomic electrons. They are called characteristic x-rays since they have energies determined by the atomic energy levels.
In interactions with matter, x-rays are ionizing radiation and produce physiological effects which are not observed with any exposure of non-ionizing radiation, such as the risk of mutations or cancer in tissue.
Gamma Rays
The term gamma ray is used to denote electromagnetic radiation from the nucleus as a part of a radioactive process. The energy of nuclear radiation is extremely high because such radiation is born in the intense conflict between the nuclear strong force and the electromagnetic force, the two strongest basic forces. The gamma ray photon may in fact be identical to an x-ray, since both are electromagnetic rays; the terms x-ray and gamma rays are statements about origin rather than implying different kinds of radiation.
In interactions with matter, gamma rays are ionizing radiation and produce physiological effects which are not observed with any exposure of non-ionizing radiation, such as the risk of mutations or cancer in tissue.
References
- ↑ CESAR. (n.d.). The electromagnetic spectrum Cesar's Booklets.
- ↑ Lucas, J. (2019, February 27). What Is Infrared? LiveScience. https://www.livescience.com/50260-infrared-radiation.html.
- ↑ HyperPhysics. (n.d.). Infrared. Electromagnetic Spectrum. http://hyperphysics.phy-astr.gsu.edu/hbase/ems3.html.