Thermogravimetric Analysis: Difference between revisions
No edit summary |
No edit summary |
||
| Line 6: | Line 6: | ||
|[[File:TGA4000.jpg|center|300px]] | |[[File:TGA4000.jpg|center|300px]] | ||
|- | |- | ||
|Ambient to 1000 °C | |'''Temperature Range:''' Ambient to 1000 °C | ||
|- | |- | ||
| Balance Precision 0.001% 0.01% | | '''Balance Precision:''' 0.001% 0.01% | ||
|- | |- | ||
|Balance Capacity 1300 mg 1500 mg | |'''Balance Capacity:''' 1300 mg 1500 mg | ||
|} | |} | ||
Revision as of 14:58, 28 June 2021
| TGA |
|---|
| Projected availability: Fall 2021 |
| Temperature Range: Ambient to 1000 °C |
| Balance Precision: 0.001% 0.01% |
| Balance Capacity: 1300 mg 1500 mg |
Thermogravimetric analysis (TGA) uses heat to force reactions and physical changes in materials[1]. TGA provides quantitative measurement of mass change in materials associated with transition and thermal degradation. TGA records change in mass from dehydration, decomposition, and oxidation of a sample with time and temperature. Characteristic thermogravimetric curves are given for specific materials and chemical compounds due to unique sequence from physicochemical reactions occurring over specific temperature ranges and heating rates. These unique characteristics are related to the molecular structure of the sample.
Principle
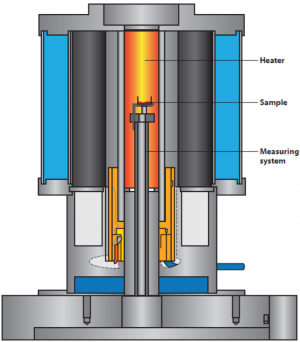
For classic thermogravimetry, a sample is usually put into a crucible out of inert material (e.g. platinum, alumina, gold…) and placed on a sensor within a furnace that can apply controlled atmospheres and temperatures[2]. The sensor is connected to a high-resolution microbalance that records the mass change during the experiment. In most thermogravimetric analyzers, compensation balances are used for this purpose. A compensation balance is a special version of a balance where an electric magnet compensates the applied mass-lab balances often work according to the same principle. While the sample sees a change in heat, the balance is usually kept in a static temperature environment in a separate chamber or at a certain distance from the sensor. The exact temperature of the sample is monitored by thermocouples that use the Seebeck-effect for precise temperature determination.
Data Output & Analysis
TGA gives a mass vs. temperature plot. The absolute value of the derivative of mass vs. temperature is known as “DTG”, and can also be plotted against temperature. The degradation temperature of samples can be found from the largest value of DTG (point of fastest degradation). The 5%, 50%, and 99% mass lost temperatures (where applicable) can be determined from the initial mass. The primary component of initial weight loss is usually evaporation of moisture and other volatiles. At larger values of weight loss weight loss the primary factors are typically pyrolysis, reduction, and desorption. While both inert ([math]\displaystyle{ N_2 }[/math] or noble gas) and oxidizing ([math]\displaystyle{ O_2 }[/math]) atmospheres can be used for TGA, the inert atmosphere is often preferred so it doesn’t interact with the sample and cause unwanted weight gain, potentially ruining any data collected.
References
- ↑ Intertek. (n.d.). Thermogravimetric Analysis (TGA) ASTM E1131, ISO 11358. https://www.intertek.com/polymers/testlopedia/tga-astm-e1131/.
- ↑ Thermogravimetric analysis - analytical method of [mg]. Linseis Messgeräte GmbH. (2020, November 26). https://www.linseis.com/en/methods/thermogravimetric-analysis/.